The International Society for Cellular Medicine (ICMS) recognizes the need to establish standards for platelet-rich plasma (PRP) procedures, preparation, techniques, and follow-up. The Society is led by clinicians with the goal of better outcomes and is dedicated to meeting the needs and interests of patients and physicians. To advance PRP (and autologous cell therapy in general), we have established the following principles to assist physicians in safe treatment, promote patient education, encourage sound clinical research, and set appropriate goals and expected outcomes.
PRP: Historical Background
PRP has been used in a variety of fields, and was first applied by M. Ferrari in 1987 for autologous transfusion in patients undergoing open heart surgery, thereby avoiding homologous transfusion. Currently, there are more than 5,200 records related to PRP in the National Center for Biotechnology Information (NCBI) in the fields of orthopedics, sports medicine, dentistry, ophthalmology, otorhinolaryngology, neurosurgery, urology, trauma repair, cosmetic, cardiothoracic, and maxillofacial surgery.
The initial popularity of PRP stemmed from its ability to serve as a safe and natural alternative to surgery, and proponents of PRP believe the process is a proto-therapeutic one that uses the body’s own growth factors to promote healing. In recent years, increased scientific research and technological advances have provided new insights into platelet function. Studies have shown that platelets contain high levels of growth factors and cytokines that can impact inflammation, postoperative blood loss, infection, osteogenesis, wounds, muscle tears, and soft tissue healing. In addition, platelets release many biologically active proteins that recruit macrophages, mesenchymal stem cells, and osteoblasts, which not only facilitate the elimination of degenerative and necrotic tissues, but also improve tissue regeneration and healing.
In the early 1990s, a number of researchers working with the musculoskeletal system began using PRP to treat tendon tissue disorders. These early medical practitioners were initially trained in how to stimulate local cell proliferation and achieve tissue repair. PRP became popular when physicians discovered that aggregating a patient’s own blood factors could achieve good clinical results. At the time, PRP was a complex procedure to perform and required additional equipment to accomplish. However, many physicians have seen that it has excellent efficacy, is simpler in its approach, and not only that, it gives better tissue viability compared to augmentation therapy.
The development of PRP therapy has relied heavily on case reports, and historically there have been few controlled trials to demonstrate the effectiveness of PRP. Of the available trials, the sample size is often too small to produce generalizable results. In addition, exogenous activation of platelets, and even appropriate candidates for the process, are not defined because there is no consensus on technique, number of injections, injection interval, number of platelets, platelet concentration at baseline, and the presence or absence of leukocytes at the time of injection, and thus further definition and evaluation is needed to address the above issues. Recently, new literature has reported the efficacy of PRP in the treatment of chronic nonhealing tendon injuries, including humeral epicondylitis, plantar fasciitis, and cartilage degeneration.
This guideline will focus on the use of PRP and its specific applications in the treatment of musculoskeletal disorders. Other areas such as dentistry, aesthetics, and wound healing will be added separately.
PRP: Definition and preparation points
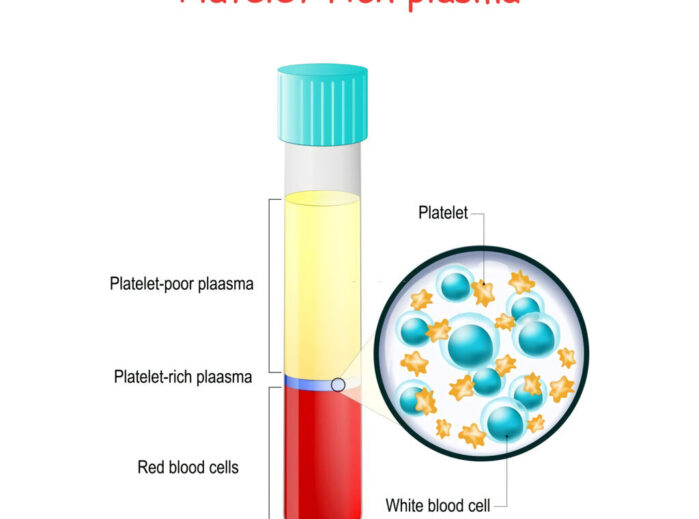
By definition, PRP must contain a higher concentration of platelets than the basal value; however, being rich in large numbers of platelets is only a very general description of PRP and does not accurately describe the variability between different types of PRP. However, there are several parameters to include in consideration regarding PRP, which include: platelet concentration above basal values, whether leukocytes are included, whether the PRP has been anticoagulated, and whether it requires exogenous activation.
Platelet count is the first variable to be considered. The absolute amount of platelets varies with the platelet concentration in the patient’s peripheral blood.PRP preparation devices can usually be divided into low (2.5-3x baseline concentration) and high (5-9x baseline concentration) systems. Intuitively, it appears that higher platelets will produce more growth factors and achieve better clinical outcomes, but this has not been established. graziani et al. suggest that the optimal concentration of PRP should be 2.5x baseline, beyond which inhibitory effects may occur. More studies are needed to confirm this.
PRP containing leukocytes will have a different biological activity compared to PRP without leukocytes. The low-fold platelet concentration system separates whole blood into two parts: one part with cellular components and the other part consisting of serum with platelets in suspension. The high magnification platelet concentration system separates whole blood into three fractions: red blood cells, serum and a brownish layer of sediment. The brownish layer of sedimentation contains platelets and white blood cells (WBCs).
WBCs can be further divided into different types, including neutrophils, monocytes/macrophages and lymphocytes. They have different roles in tissue repair. Neutrophils are phagocytic and contain more than 40 hydrolytic enzymes; their activation leads to phagocytosis of debris debris, release of oxygen radicals and proteases. The release of toxic molecules from neutrophils can cause secondary muscle damage. It has not been determined whether neutrophils have a positive or negative effect on acutely or chronically injured soft tissues.
Macrophages are the organizing form of circulating monocytes. Their role is to remove debris debris and they mainly exert phagocytosis. They also play a balancing role in pro- and anti-inflammation. Since different leukocytes cannot be isolated from PRP, the absence of macrophages may be more severe than the secondary damage caused by macrophages. More research needs to be done in this area as well.
When blood is drawn, many PRP kits come with an anticoagulant to prevent blood clotting. Most kits use the anticoagulant glucose citrate (ACD) to inhibit clotting. ACD chelates calcium ions and prevents clotting proteins from initiating clotting factors. The addition of citric acid also makes the blood more acidic than physiological. Since some growth factors are affected by tissue pH, some scholars recommend buffering PRP back into the physiological range prior to injection.
Activation of PRP prior to injection is another factor that needs to be explored in depth. prP can be activated by exogenous thrombin, calcium chloride, or mechanical trauma. Once PRP is activated, a fibrin network begins to form, causing the plasma to coagulate and form a fibrin clot or membrane. If PRP is over-activated, a bivalent fibrin network will form, an unstable network. If PRP is activated in a more physiologically appropriate manner, a stable tetravalent network will form, which can improve the ability to trap cells and growth factors. Although this is useful for surgical procedures, it is also undesirable to inject PRP that is too viscous into soft tissue.
Activation results in rapid release of growth factors, with 90% of growth factors released within 10 minutes. Many growth factors have short half-lives, so activating PRP at the time of injection or just prior to injection may have the greatest positive effect. The different half-lives of growth factors form the PRP used at different times after activation. most commercial PRP kits do not activate PRP. some kits replace the calcium chelated by glucose citrate, resulting in a more physiologic state. By tissue injection, the use of unactivated PRP may result in a more normal physiological activation.
To avoid inadvertent platelet activation, most methods use a large bore syringe needle (>22) to draw blood and reinject PRP. In addition, there are different requirements for centrifugation speed and centrifugation time. Some centrifuges provide special braking mechanisms to prevent accidental activation. The optimal protocol to prevent accidental activation is not yet known. Collagen is a natural activator of PRP, so when PRP is applied in soft tissue, it does not require exogenous activation.
Once activation has occurred at the injection site, the inflammatory response initiated by the release of growth factors lasts approximately 3 days. Fibroblasts accumulate at the injection site, which marks the beginning of a healing and proliferative phase that lasts several weeks. Thereafter, remodeling of the collagen matrix deposited in the fibroblasts begins. The remodeling phase, which forms mature tissue, lasts approximately 6 months. The formation of new tissue goes through all three of these phases in order to form long-term stable tissue.
Platelet Rich Plasma (PRP): Physician Certification
PRP injections should be performed by a nationally board-certified clinician. Successful and safe application of PRP therapy requires that the physician has a thorough knowledge of the diagnosis of the disease, standard therapies, efficacy, risks, contraindications and preparation methods, and is able to select the right patient for application of the therapy in the right situation.
Physicians who perform PRP injections should be certified to ACGME standards and maintain comprehensive and specific continuing medical education credits in the musculoskeletal system. New discoveries in diagnosis, treatment, anatomy and radiology in the area of chronic pain or acute neuromusculoskeletal injuries are particularly encouraged. CME-certified new findings on PRP research, steps in the use of PRP, and the clinical application of PRP are strongly encouraged.
Physicians who clinically apply PRP injections for treatment should be familiar with the relevant literature, as well as the usual diagnostic treatments considered for PRP treatment, and be aware of the advantages, risks, and injection methods of the approach, and adhere to ICMS / AMSSM guidelines when handling and performing PRP. Currently, there is no regulatory body that authorizes the application or implementation of PRP, which is considered to be the practice of medicine.
Indications for PRP
For any skeletal muscle disorder, a complete history and examination are important for the differential diagnosis. It is also important to review previous “failed” diagnostic treatments to ensure proper management. Currently, PRP is often considered an elective treatment for subacute and chronic conditions. In general, acute injury healing slows or stops after 6-12 weeks. If a patient’s condition does not improve for more than 6 weeks, the healing process is likely to have stalled. In cases of overmedication or multiple treatments, it is extremely challenging to distinguish the transitional state between the subacute and acute phases.
We present some common orthopedic indications for PRP and provide some preliminary but not comprehensive summaries of clinical information for each indication. We believe that evidence-based medicine is a valid tool, but for patients, multiple treatments are often applied simultaneously, making it difficult to assess the role of PRP solely quantitatively in RDBCT clinical trials.
Tendinopathy
Tendinopathy is a group of degenerative tendinopathies characterized by a chronic loss of tendon collagen, tissue integrity, stability and strength. Tendinopathy is not an inflammatory state and lacks inflammatory cells in the tissue sections. The causes of this lesion are multifactorial, while age, injury, repetitive stress, neurological, vascular and hormonal have possible causative factors. Whereas tendinopathy is almost ubiquitous with age, pain and dysfunction usually occur only when sufficient stress is applied to the degenerated tendon.
Both basic research and animal studies support the use of PRP in the treatment of tendinopathies. Experimental studies have demonstrated its effects on tendon cell proliferation, collagen deposition, and endogenous growth factors. Very promising results have also been achieved with PRP in studies of animal injury models of surgical injury. However, the RDBPCT clinical trial still lacks clear population-based evidence of positive significance, and several other clinical trials have shown negative results, with a series of successful case reports by Mischra and Barnett for PRP in intractable tennis elbow and plantar tendinitis, respectively.
We encourage more in-depth studies on technique, number of injections, injection intervals, platelet counts, baseline platelet concentrations, presence of leukocytes in injections, activation of exogenously injected platelets, common methods of outcome assessment (e.g., VISA scores), and patient selection.
Ligament Sprains
Most human ligament studies to date have incorporated pre-surgical cruciate ligament reconstruction. Overall, the findings have shown that this approach improves pain, healing rates, and graft quality. There is still a lack of support in the sports medicine literature for non-surgical treatment, but some experts believe that non-surgical treatment may improve healing time, reduce pain, and recovery time. We encourage more research in this area.
Muscle strains
Muscle strains are very common functional pain, especially in athletes. Muscle tissue is rich in blood supply and recovery time is approximately 8 times faster than ligament injuries with general treatment and routine care. If the subacute or chronic state continues to progress, PRP treatment may be considered. The application of PRP therapy in acute trauma to promote functional recovery is very rare and there is still a lack of strong evidence to support this practice. If PRP techniques are applied in the population on a large scale, it is not known whether they are clinically, functionally, or related to their effects in restoring motor capacity. The application of aspiration plus drug injection and PRP injection for the treatment of ossifying myositis is an area worthy of investigation.
Arthritis
Arthritis (OA) is a chronic degenerative lesion of hyaline cartilage. Arthritis is highly prevalent, painful and costly. Its serious consequences are of great concern to the individual human being and to the population as a whole, especially in the elderly. Once a patient progresses from the cartilage degeneration stage to the development of a significant clinical phenotype, there are few effective interventions available to improve symptoms. Since the body lacks the appropriate healing response to the degenerative stage, growth factor and cytokine injections are wise choices. Studies have shown that good results are generally observed with the application of PRP for arthritis, and Kon et al. suggest that PRP treatment may also improve joint function.Whether the effects of PRP are mediated by local paracrine factors that reduce pain, by the formation of new hyaline or fibrocartilage, or by a combination of both, or neither, is unknown at this time. We encourage further high-quality studies with anterior/posterior imaging and joint fluid analysis to help elucidate the possible mechanisms of PRP for OA. Animal model studies have shown that PRP treatment also significantly improves the healing efficiency of meniscus, glenoid labrum, and OCD-induced defects, but population-based evidence is still lacking.
Intervertebral discs
Although various animal models have shown encouraging results in PRP treatment, there is still a lack of population-based evidence. Discography has shown that placement of PRP at the intervertebral disc inevitably causes damage to the disc that is potentially permanent. Because of the close proximity of important neural structures to the posterior fibrous annulus, the clinical preference is for a CT or fluoroscopic-guided approach to place some regenerative factors into the intervertebral disc.
Nerve
Surgical release/decompression (neurolysis) has traditionally been used for neuropathies that have failed to respond to “conservative treatment”. With the development of musculoskeletal ultrasonography, it is now possible to clearly visualize the peripheral nerve and its adjacent structures. Much clinical experience has been gained in the application of different methods to perform percutaneous nerve release. Although there is insufficient evidence to support the use of PRP in this regard, if ischemic injury to the nerve due to scar tissue adhesions occurs, PRP theoretically has a role to play during percutaneous treatment, and further investigation is encouraged.
Bone Non-union
Osteonecrosis is a very unfavorable but rare complication in fracture management. The role of PRP in acute fractures in humans has not been well evaluated, and healing rates have not been confirmed. Further studies of bone discontinuity are valuable not only for the accumulation of relevant literature, but also for patients with prolonged healing process, perhaps allowing this group of patients to avoid the use of bone stimulators.
Platelet-rich plasma: contraindications
Absolute contraindications
- Platelet dysfunction syndrome
- Severe thrombocytopenia
- Hemodynamic instability
- Sepsis
- Localized infection
- Patients who do not want to accept the risk
Relative contraindications
- NSAID drug discontinuation not exceeding 48 hours
- Previous corticosteroid injections in the affected area within 1 month
- Systemic corticosteroid therapy discontinued for less than 2 weeks
- Smoking
- Recent fever or other illness
- Cancer – especially of the hematopoietic or skeletal system
- Hemoglobin < 10 g/dl
- Platelet count < 105/ul
PRP: Operating procedures, technical points and safety regulations
Procedure and technical points
In general, PRP transplantation can be performed with only one physician and one assistant, maintaining sterility and recording ultrasound images (if feasible).
Precautions before the procedure:
1) There should be a specific indication that corresponds to the sign, and the diagnosis should be confirmed by imaging such as X-ray, ultrasound, MRI or CT.
- Appropriate preoperative education and discussion and signed informed consent.
- Exclusion of the above contraindications.
- Preoperative administration of analgesic or anti-anxiety medication (if feasible).
Preparation for transplantation
- Arrange the patient in a comfortable sitting or lying position.
- Use and handle disposable sterile injectables and needles appropriately.
- Take an appropriate amount of venous blood under sterile conditions according to the procedure.
- Single blood draw to reduce the chance of PRP activation.
- If the blood vessel is penetrated, blood flow is poor, the needle penetrates the vein or multiple attempts at one site are ineffective, a different site should be considered for blood sampling.
- Consider using ultrasound-guided blood draws if the patient has difficulty drawing blood.
- Transfer venous blood to a centrifuge under aseptic conditions. prp should be obtained from a separate device used for autologous blood separation. Prefer a closed system to prevent exposure of blood and cellular fractions to air and to minimize tissue manipulation.
- If multiple patients are being prepared for transplantation at the same time, they should be properly labeled to prevent cross-contamination or misuse.
Imaging Guidance
1) Use CT, X-ray fluoroscopy or ultrasound for real-time imaging guidance when injecting PRP.
2) Factors to consider before using ultrasound:
- Sterile gel. We recommend this gel for longer procedures such as intra-articular and peri-spinal injections. The widespread use of this gel does not help to reduce the infection rate, and for simple soft tissue injections, reasonable use of sterile technique is sufficient.
- Sterile probe coverage. We recommend its use during longer procedures (e.g., percutaneous puncture tendotomy, etc.). Clean the probe before and after use and adhere to aseptic practice. Aseptic trauma products (dressings) or sterile gloves can also be used to cover the probe, and the above have been used successfully in the community.
- Mark the probe and injection needle entry location before final skin cleaning.
PRP injection
- Have the patient in an appropriate and comfortable position, maintaining sterility and access to the injection port position.
- Prepare the relevant items needed for the injection (PRP, additives, 4*4s, needle, US gel) and place them on a sterile table near the physician for easy access.
- Local disinfection, laying of towels and establishment of sterile area.
- Pay attention to aseptic practice when using local anesthesia. See discussion of the effects of anesthesia on PRP above. Subcutaneous local infiltration, or nerve block anesthesia (for longer operations such as tendotomy) may also be used.
- If ultrasound is used, the gel should be applied to the same site as the pre-marked site.
- The complete injection procedure is recorded on real-time video.
- Apply a dressing or bandage to protect the needle entry site.
Post-injection
- Monitor for post-operative complications (vasovagal reactions are most common)
- Inform the patient of postoperative instructions, precautions and emergency contact information.
- The protocol has not yet reached consensus on postoperative patient braking or activity, and specific recommendations are expected to be given when the protocol is disseminated.
- Give postoperative analgesics. The use of NSAIDs is prohibited until the patient has recovered, relieved pain, and achieved functional integrity or a stable level.
- Disinfect contaminated areas after one patient use and before the next in accordance with Occupational Safety and Health regulations (OSHA).
- The procedure should be documented in detail in the form of notes including: date, pre/post diagnosis, name of the procedure, performing physician, assistant, anesthesia, short description of the operation, description of the graft preparation, description of the procedure including instructions and instruments.
Follow-up visits
- Patients usually return in 2-6 weeks for follow-up visits for recovery of pain, function, injection location, discussion of precautions and subsequent rehab arrangements.
- Patient response is recorded and evaluated using validated outcome assessment methods such as Nirschl, VISA, etc.
- Complications, reactions and all other relevant data should be entered into the ICMS follow up system.
- The need for reinjection should be decided by the patient and based on functional results. We do not give recommendations for specific number of injections and sites (the decision should be made by the condition and the patient).
Safety instructions:
- Full precautions should be taken during the procedure and subsequent processes.
- Infection: PRP is antimicrobial and effective against most bacterial species except Klebsiella, Enterococcus and Pseudomonas. Standard skin disinfection should be performed prior to injection.
- This is a completely autologous graft, which eliminates concerns of infectious disease unless the graft is contaminated.
Patient risk
- Infection
- Bleeding
- Nerve damage
- Pain
- Ineffective
- Amputation and death, very rare but possible.