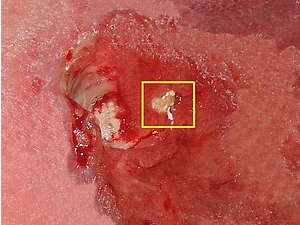
Anterior cruciate ligament (ACL) reconstruction is a common surgical procedure used to treat ACL injuries. One of the most common graft options is the patellar tendon graft, which involves harvesting the central third of the patellar tendon to reconstruct the ACL. However, this procedure can result in significant donor site morbidity, including pain, weakness, and decreased range of motion.
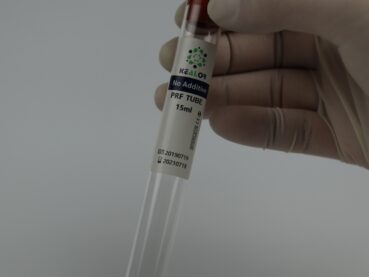
Platelet-rich plasma (PRP) therapy is a non-surgical treatment that uses the patient’s own blood to promote healing and reduce inflammation. It contains a concentrated amount of growth factors and cytokines that can stimulate tissue repair and regeneration.
Several studies have investigated the use of PRP to reduce donor site morbidity in patients undergoing patellar tendon graft harvesting for ACL reconstruction. A systematic review and meta-analysis published in the journal Knee Surgery, Sports Traumatology, Arthroscopy in 2020 found that PRP reduced pain and swelling at the donor site and improved knee function compared to a control group.
Another study published in the American Journal of Sports Medicine in 2017 found that the use of PRP at the patellar tendon harvest site improved postoperative function and reduced pain and stiffness compared to a control group.
The mechanism behind the effectiveness of PRP in reducing donor site morbidity is not yet fully understood, but it is believed to be related to its ability to enhance tissue regeneration and reduce inflammation. By delivering growth factors and cytokines directly to the injured tissue, PRP can promote healing and reduce pain and inflammation.
In conclusion, the use of PRP therapy appears to be a promising option for reducing donor site morbidity in patients undergoing patellar tendon graft harvesting for ACL reconstruction. However, more research is needed to fully understand its effectiveness and determine the optimal protocol for its use. Patients should always consult with their healthcare provider to determine the best treatment options for their individual needs.